List Of Eye Drops Recall 2024
List Of Eye Drops Recall 2024. Product recalls are becoming more common in the u.s.: [1/31/2024] fda is warning consumers not to purchase or use south moon, rebright or fivfivgo eye drops because of the potential risk of eye infection.
1, 2024, at 9:46 a.m. The fda updated the list of recalled items on oct.
Is Voluntarily Recalling Two Lots Of Equate Hydration Pf Lubricating Eye Drops.
The fda updated the list of recalled items on oct.
If You’re Dealing With Dry, Irritated Eyes, You May Rely On Artificial Tears To Find Relief.
Fda warns eye drops may cause infection.
59,825 2022 To 2024 Grand Cherokee 4Xe Suvs Built Between July 23, 2021, And December 5, 2023.
March 8, 2024, 4:00 am pst.
Images References :
 Source: www.sportskeeda.com
Source: www.sportskeeda.com
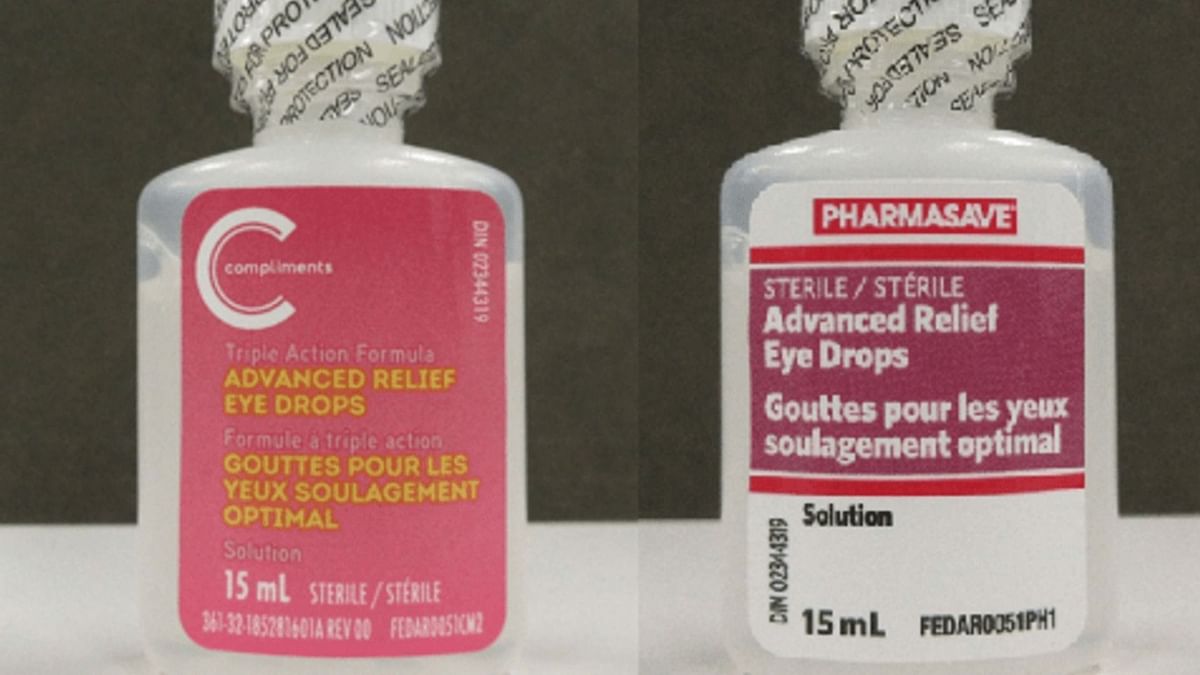
Eye Drops Recall 2022 Everything to know about Pharmasave and, The food and drug administration (fda) is warning people not to use two types of eye drops that may contain bacterial contamination, fungal. Delsam pharma artificial tears lubricant eye.
 Source: www.sportskeeda.com
Source: www.sportskeeda.com
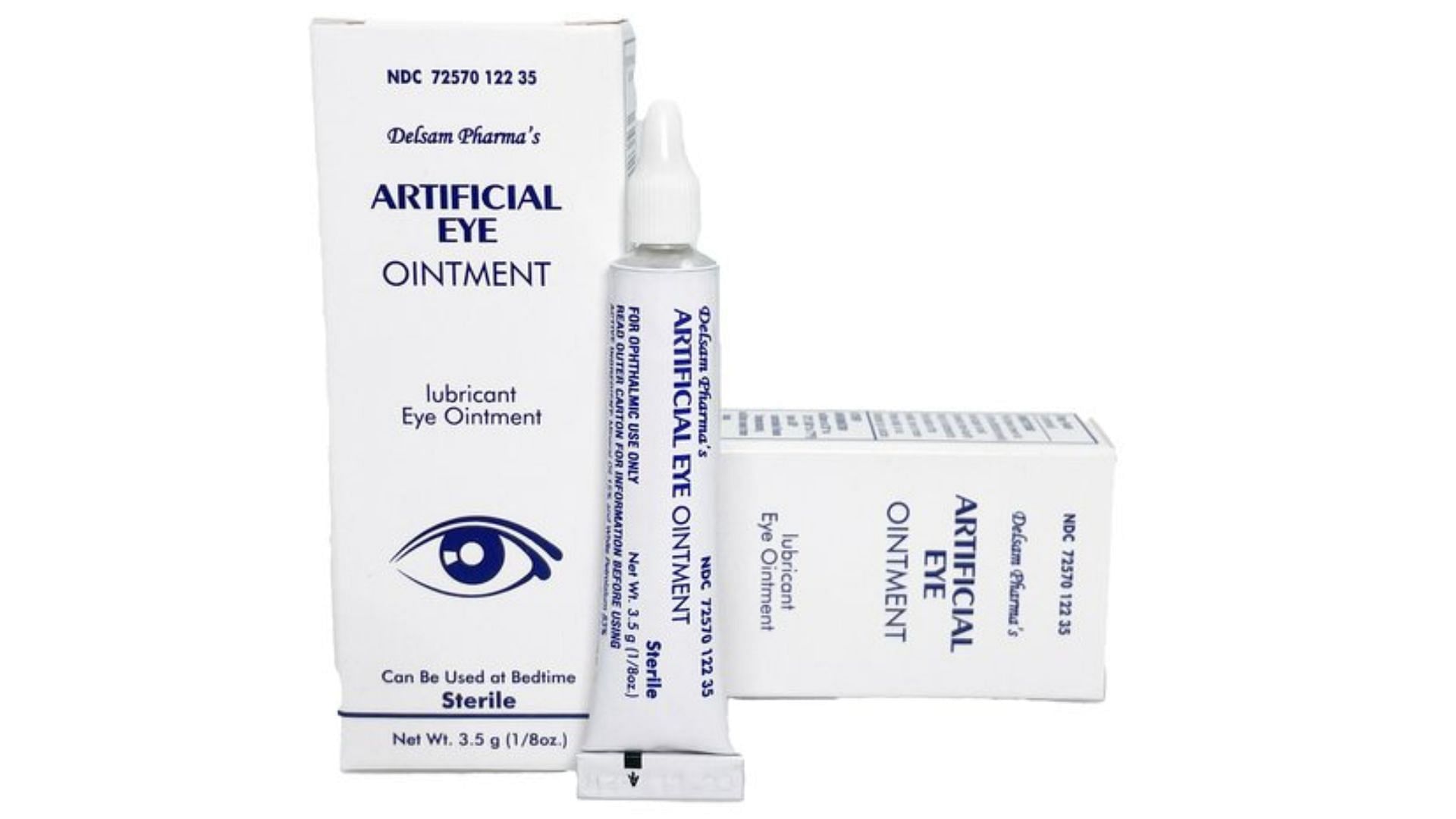
What eye drops are contaminated? FDA expands warning recall list, Is voluntarily recalling two lots of equate hydration pf lubricating eye drops. If you’re dealing with dry, irritated eyes, you may rely on artificial tears to find relief.
 Source: www.aarp.org
Source: www.aarp.org
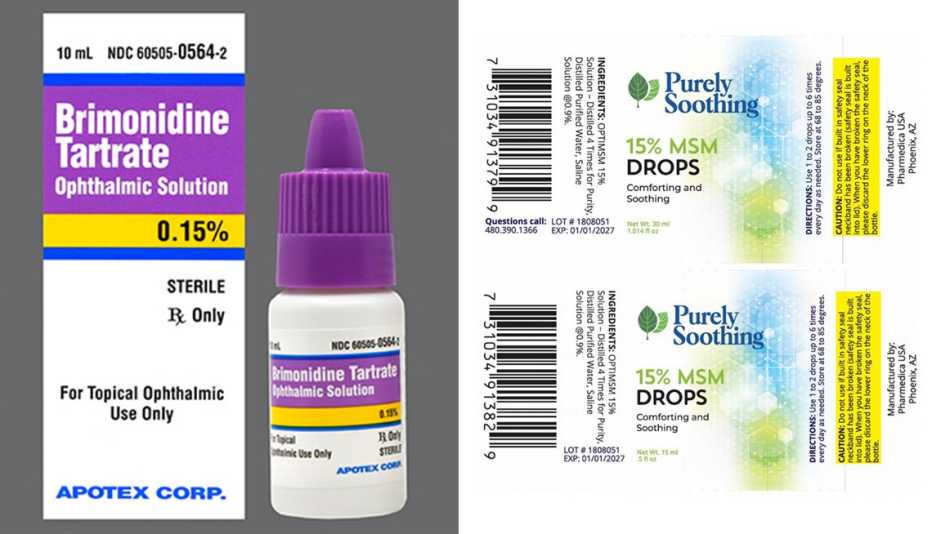
2 More Eye Drop Brands Recalled Due to Safety Risks, 1, 2024, at 9:46 a.m. The food and drug administration issued an alert on friday flagging 26 eye care products including eyedrops and gels from cvs health, leader (cardinal health),.
 Source: www.sportskeeda.com
Source: www.sportskeeda.com
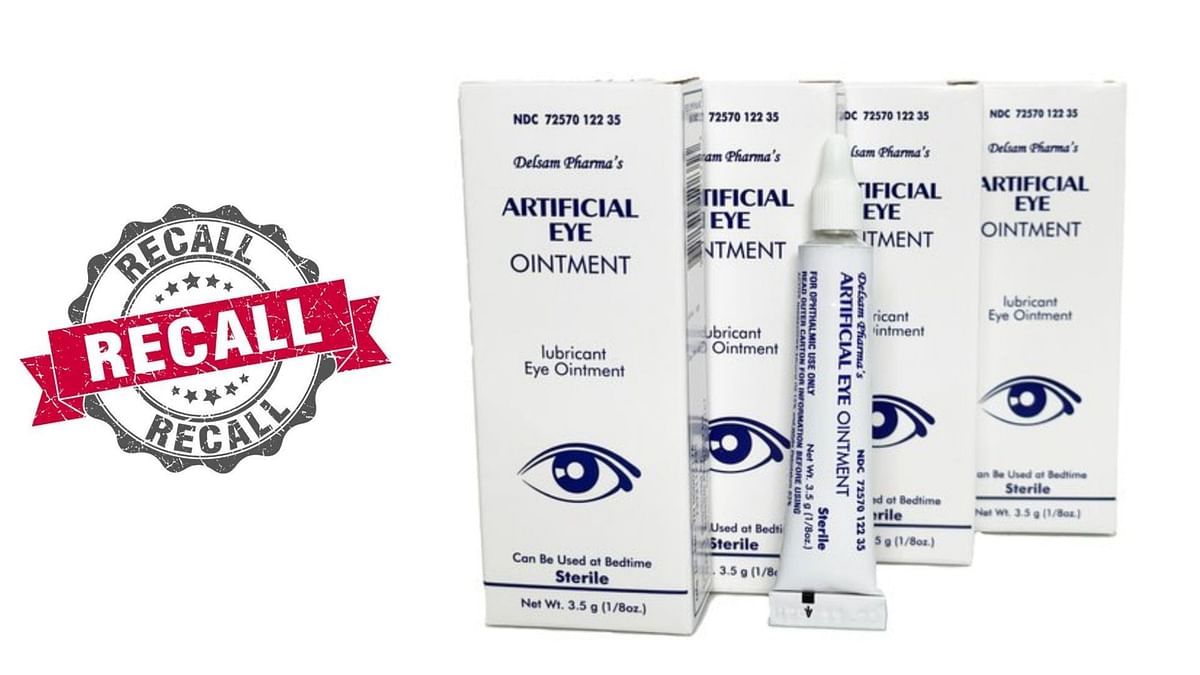
What eye drops are contaminated? FDA expands warning recall list, Per the fda, kilitch healthcare india limited voluntarily recalls 27 eye drop items, including those distributed by cvs, rite aid, and target, due to potential safety. The new recall is one of multiple eye product recalls within the past year.
 Source: www.hitc.com
Source: www.hitc.com
What brands of eye drops are being recalled in 2023?, By aria bendix and rob wile. 1, 2024, at 9:46 a.m.
 Source: www.goodto.com
Source: www.goodto.com
Eye drop recall 2023 The full list of brands that have been recalled, Delsam pharma artificial tears lubricant eye. Per the fda, kilitch healthcare india limited voluntarily recalls 27 eye drop items, including those distributed by cvs, rite aid, and target, due to potential safety.
 Source: www.denver7.com
Source: www.denver7.com
FDA expands recall of eye drop products after reports of eye infections, In 2023, certain eye drops were recalled due to. Global pharma healthcare recalled artificial tears lubricant eye drops, distributed by ezricare and.
 Source: ndclist.com
Source: ndclist.com
FDA Recalls NDC 49348037 Sunmark Eye Drops Original Formula Liquid, The fda says the eye drops are marketed under retailers and brands walmart, cvs health, rite aid, target up&up, leader, rugby, and velocity pharma,. Published 1:50 pm edt, fri may 19, 2023.
 Source: www.lawsuit-information-center.com
Source: www.lawsuit-information-center.com
New Eye Drops Recall Lawsuit — Lawsuit Information Center, The food and drug administration issued an alert on friday flagging 26 eye care products including eyedrops and gels from cvs health, leader (cardinal health),. Per the fda, kilitch healthcare india limited voluntarily recalls 27 eye drop items, including those distributed by cvs, rite aid, and target, due to potential safety.
 Source: www.elevate.in
Source: www.elevate.in
Eye Drops Recall 2023 List Every Brand Recalled Over, 49 OFF, Jeep will inform owners of the affected cars to see an authorized. The food and drug administration (fda) is warning people not to use two types of eye drops that may contain bacterial contamination, fungal.
Check For Recalls Or Talk To Your Pharmacist First.
Jeep will inform owners of the affected cars to see an authorized.
Food And Drug Administration Has Issued Another Warning To Consumers Not To Purchase Certain Eyedrop Brands Due To Risk Of Eye Infection.
The food and drug administration issued an alert on friday flagging 26 eye care products including eyedrops and gels from cvs health, leader (cardinal health),.
[1/31/2024] Fda Is Warning Consumers Not To Purchase Or Use South Moon, Rebright Or Fivfivgo Eye Drops Because Of The Potential Risk Of Eye Infection.
In 2023, certain eye drops were recalled due to.
Posted in 2024